REJOINT
Regeneration and pathophysiology of joints
This team is composed of 46 people (36 full-time equivalent) including 15 senior researchers with Research Supervisor Qualifications (HDR). The team is headed by J. Guicheux and F. Blanchard as deputy head.
The REJOINT team is a translational and transdiciplinary team that focuses on the pathophysiology and aging of the joints from their basic aspects (cell fate, tissue modeling, senescence, autophagy, inflammation) to more applied aspects (rheumatoid arthritis, osteoarthritis, disc degenerative disease, osteochondral defects). The REJOINT team studies the fundamental processes of stem cell fate and differentiation during development and growth of cartilage and intervertebral disc. This team will also support the development of (i)
bio-inspired hydrogels for drug delivery systems and bioprinting, and (ii) strategies to exploit the properties of extracellular vesicles, mesenchymal or pluripotent stem cells for the regenerative medicine of cartilages and discs. To address this ambitious research program REJOINT is organized around 6 groups
Group 1 - BIODIV: Stem cells and axial skeleton development
Leader: A. Camus 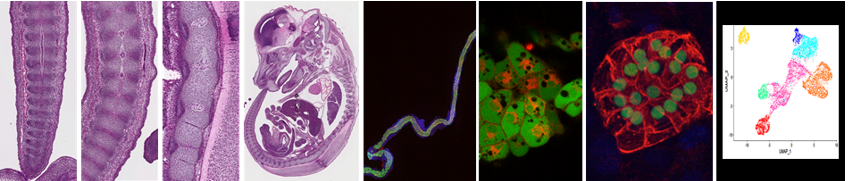
This theme involves 1 senior scientist (A. Camus-DR2 CNRS)
Groupe 2 AGE-OA: Aging and OsteoArthritis Pathophysiology
Leader: Claire Vinatier 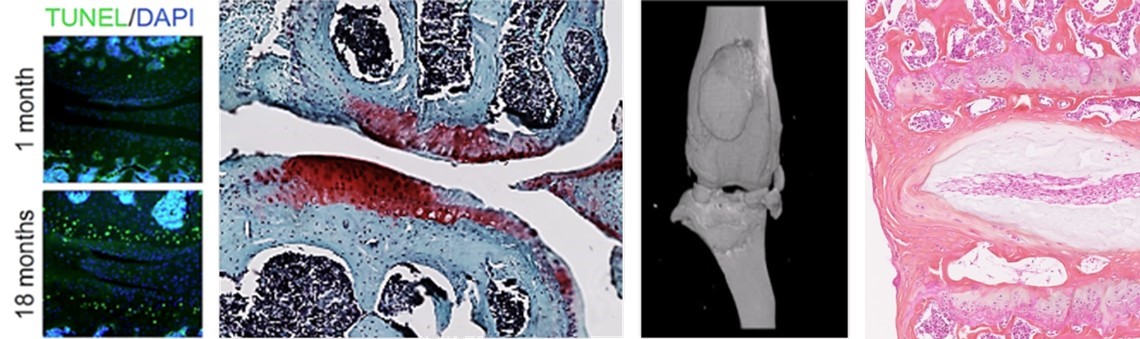
This group involves 3 senior scientist (C. Vinatier, MCU, A. Galvani, MCU A. Fouasson-Chailloux, PHU), 1 senior postdoc (R. Guiho), 2 junior postdoc (A. Marano, A Rousseliere), 3 PhD student (M. Izco Cubero, M. Raji, S. Hussein), 1 M2 student.
Program 1. Age-related mechanisms and metabolism dysregulation in osteoarthritis (leader: C. Vinatier)
Osteoarthritis, the most prevalent inflammatory joint disease with no cure to date, is multifactorial and heterogeneous. Aging is one of the main risk factors for osteoarthritis (OA). Paradoxically, the role of aging on chondrocyte homeostasis and alterations in their metabolism are only partially known. Our main goals are, (i) to decipher the role of aging-associated mechanisms in the appearance of OA, (ii) to explore the role of anti-aging factors in articular cartilage aging and OA pathophysiology (iii) to identify new molecules that target chondrocytes homeostasis and mitochondrial metabolism.
Program 2. Cellular senescence in spinal OA (leader: R.Guiho)
Like peripheral OA, the pathophysiology of Intervertebral Disc Degeneration (IVDD) remains not yet fully understood and only palliative treatments exist to limit pain. Recent studies have suggested a central role for the senescent disc cells and their associated secretory phenotype in IVDD onset and perpetuation. Our objectives are (i) to better understand the influence of this cellular state in IVDD, (ii) to decipher the mechanisms involved in senescence occurrence, in order to (iii) consider senescence targeting as a new therapeutic avenue in IVDD.
Group 3 - T-Syno : Tendon and Synovial pathophysiology
Leader: F. Blanchard 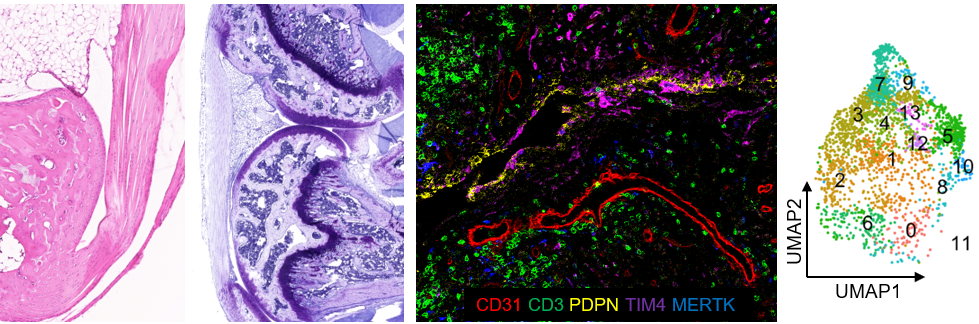
This group involves 1 senior scientist (F. Blanchard DR2 Inserm), 2 University/Hospital Professors (B. Le Goff PU-PH, C. Darrieutort PU-PH),1 PhD student ( B de Maleprade), 1 research engineer (P. Humbert) and 1 M2 student
Program 1. Therapeutic targets in synovitis (leaders: F. Blanchard and B. Le Goff)
Inflammation of the synovial membrane is the hallmark of many chronic inflammatory rheumatisms such as rheumatoid arthritis (RA) or pigmented villonodular synovitis (PVNS). Macrophages and fibroblasts play key roles in synovitis, but their crosstalk is still unclear. Our goals are (i) to better characterize synovial cells in rheumatisms, (ii) to identify pathogenic inflammatory versus protective macrophage and fibroblast subsets, and (iii) bring the in vivo proof of concept that targeting specific synovial cells or pathways may be of therapeutic potential.
Program 2. Therapeutic targets in tendinopathies (leader: C. Darrieutort)
Tendinopathies are a common medical problem occurring in and around tendons in response to overuse. Since the tendon has low vascularization and low cellularity, its natural regeneration/repair capacities are limited. Our goals are (i) to better characterize the different cells (tenocytes, macrophages etc) involved in tendon healing, (ii) their interaction in space and time and (iii) novel therapeutic targets
Group 4 - STRATOA: Immune cells and osteoarthritis personalized therapies
Leader: M.A. Boutet
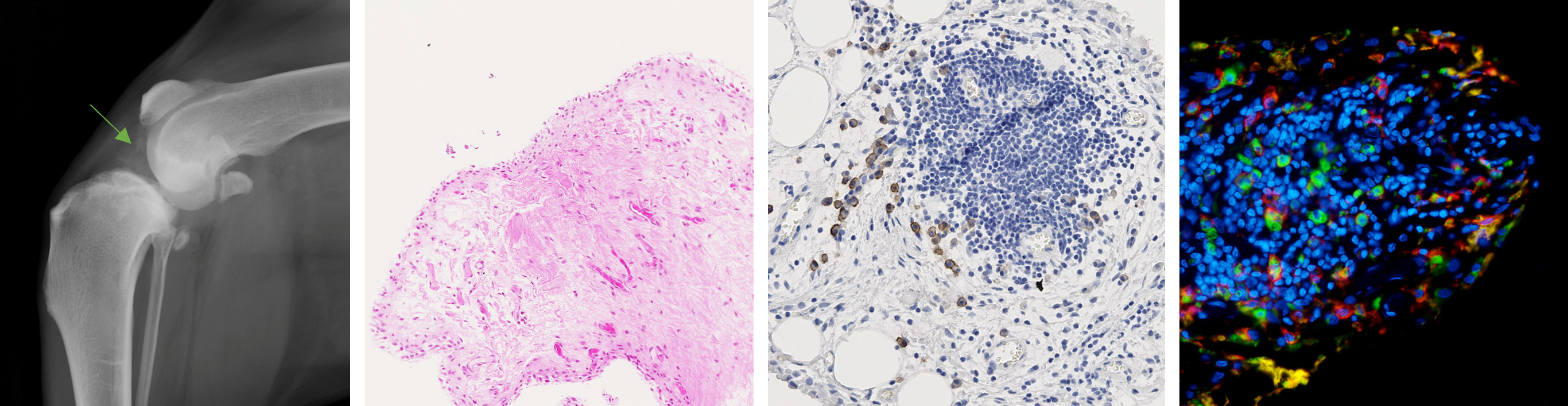
This group involves 1 ATIP-Avenir junior group leader (M-A. Boutet), 1 PhD student ( M Le Mercier), 1 postdoc (A. Cardon), 2 IE (A. Dumont, J De Lima).
Osteoarthritis (OA) is a very heterogeneous disease driven by a large variety of factors, but this variability remains poorly considered in OA translational and clinical research. Importantly, OA synovial tissue presents significant changes, even before cartilage degradation. Synovitis has been correlated with OA severity and therefore represents a relevant target for therapeutic intervention. Our aims are (i) to characterize the synovial heterogeneity and define a relevant human and canine patients stratification, (ii) validate innovative tools to efficiently modulate innate immune cells, such as macrophages, for personalized treatment of OA, and (iii) understand the broader involvement of the immune system at both the local and systemic levels in the context of OA.
Main collaborators: C. Pitzalis and A. Nerviani, A. Mantovani and B. Bottazzi, I. Mattiola and A. Diefenbach
Group 5 - BIOMAX: Bio-inspired material concepts
Leader: V. Delplace
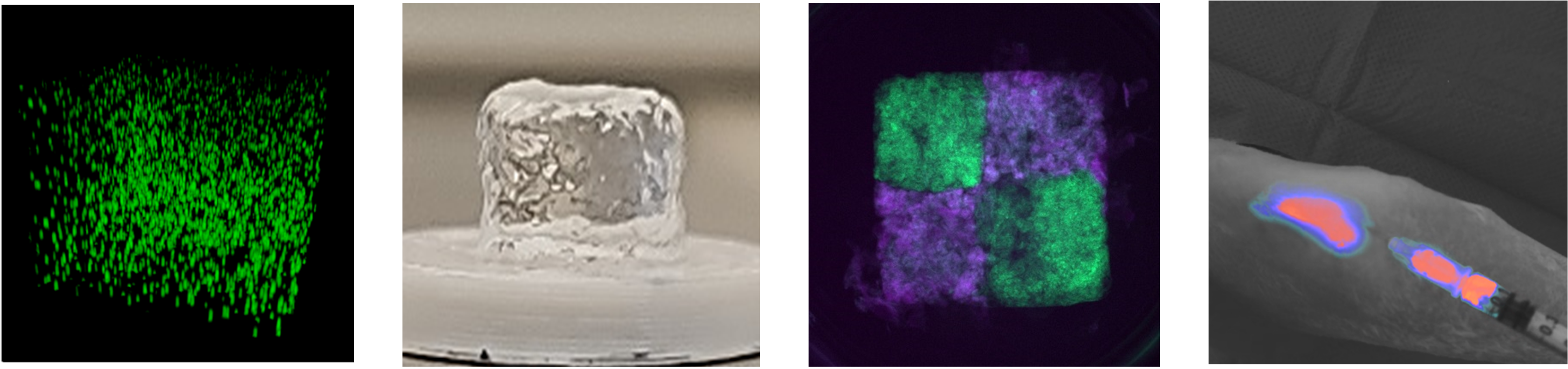
This theme involves 1 junior scientist (V. Delplace CRCN Inserm), 2 University/Hospital Professors (Y. Maugars, A Paré), 1 postdoc (J. Babilotte), 1 PhD student ( H. Tewari) and 1 IE (L. Terriac).
Biomaterials are now considered key elements in the success of innovative therapies and modeling approaches. In particular, researchers are progressively unravelling the major role of cell-material interactions on cell behavior, properties and fate. While it paves the way for material-guided therapies and advanced tissue engineering, it calls for the development of tunable material platforms and application-specific scaffold design. In the BIOMAX group, we are dedicated to tackling major challenges in the field of biofabrication and drug/cell delivery, with the development of innovative biomaterial concepts. Our research is centered on the use of new material chemistry tools for the design of "4-D bioinks", anisotropic materials, and mechanically resilient scaffolds, strongly inspired by the biological needs in the context of articular diseases.
Group 6 - HEAL: Hydrogels and joint translational research
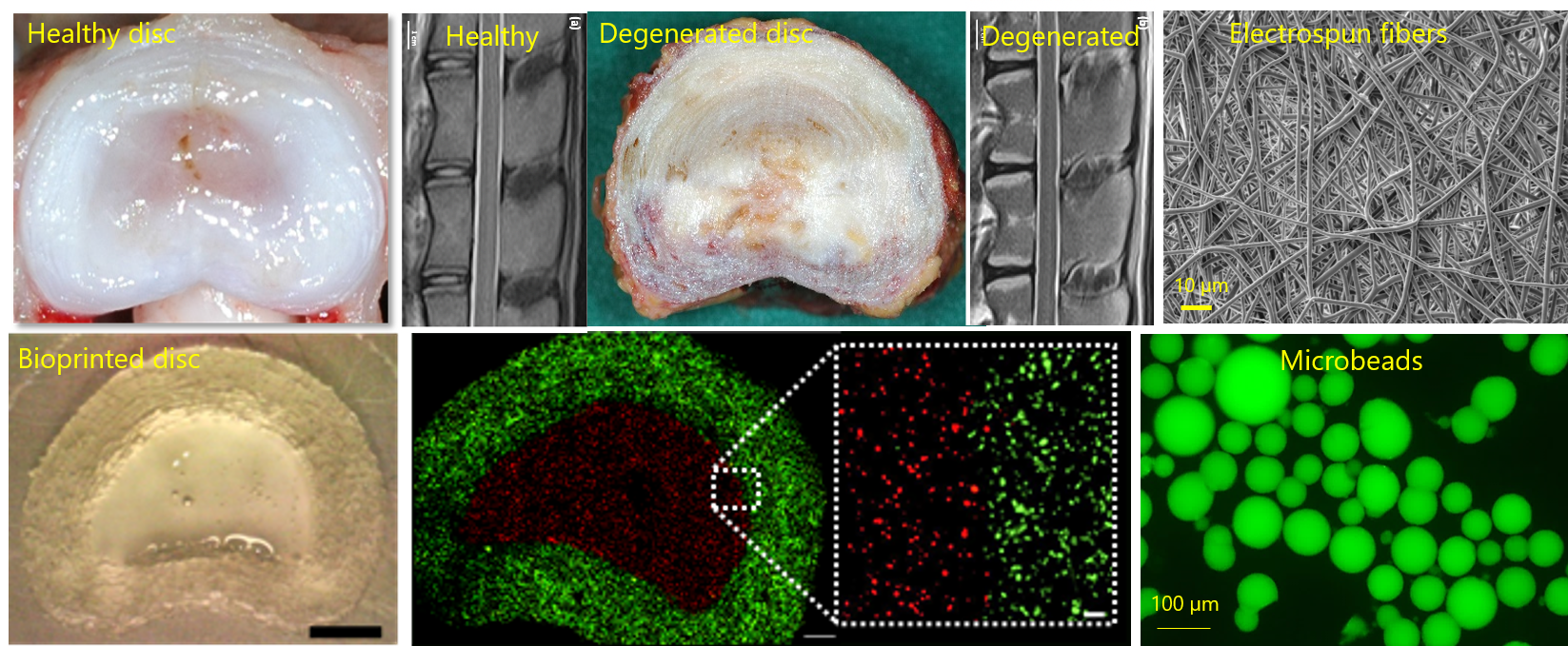
Leader: C. Le Visage
This group involves 1 senior scientist (C. Le Visage DR2 Inserm) , 5 University/Hospital Professors (J Clouet (PU-PH), M Fusellier (PU ONIRIS), A. Hamel (PU-PH), C. Decante (MCU-PH), F. Nativel (PHU)), 2 research engineer (C. Boyer, F. Etienne), 2 post-doctoral scientists (M. Chaaban, H Genedy), 1 PhD student (B. Laoulaou), 1 Erasmus intern (G. Quispe) and 1 Master student.
We develop injectable carriers of biologics for cartilage and intervertebral (IVD) regeneration, with an emphasis on i) paracrine effect of exogenous Mesenchymal Stem/stromal Cells (MSCs) and extracellular vesicles (EVs), and on endogenous repair using soluble molecules (cytokines, miRNA). The carriers include nano- and micro-sized alginate hydrogels (collab. INRAE, Nantes), electrospun fibers (collab. IBMM, Montpellier), and lipid nanocapsules (collab. MINT, Angers). The efficacy of these different strategies is evaluated in vitro using human cell lines, or primary chondrocytes and disc cells. We recently set up an ex vivo culture of sheep IVDs, and we also develop a bioprinted model of IVD (collaboration AO Foundation, Switzerland) as an alternate ex vivo model. Evaluations are then conducted in various animal models (Vet school ONIRIS), with an emphasis on innovative imaging tools (MRI, microCT), before embarking on clinical trials (EU-H2020 IPSPINE project, Region DISCODOG project, ANR NEMESIS project).
Group 7 - E-Pain: Pain and Ethics
Leader: J. Nizard
This group involves 4 University/Hospital Professors (J. Nizard, PU-PH), A Levesque (PH), G. Durand, MCU, S. Acapo (AAU, PhD) and 6 PhD student (M. Al Medawar, M. Brier, M. Chaillot, E. Eden, H. Niang, M Voiron)
Program 1.
Improve the methods used to evaluate therapies for acute and chronic pain.
Improve the efficacy of pharmacological and non-pharmacological therapies for pain associated with osteoarticular diseases, including specific syndroms such as complex regional pain syndrom and pelvic and perineal pain.
Program 2.
Improve the consideration of ethical aspects in research, in connection with the Master’s program Ethics at Nantes University (The leader of group 7 is responsible for the “Health track” of this Master’s program.
Implement research projects on the ethical aspects of pain management in humans (particularly concerning their decision-making autonomy and access to expert care facilities, and in animals.




