Research facilities
ATLAN
ATLAN (Analyses TissuLaire Angers-Nantes) est un regroupement de plateforme multisite régional composé des plateformes SC3M (Service Commun de Microscopie électronique, Microcaractérisation et Morpho-histologie-imagerie fonctionnelle) et HiMolA (Histologie Moléculaire Angers).
La structure ATLAN a pour but d'améliorer la visibilité de nos plateformes partageant un fonctionnement commun, et permettre aux utilisateurs d'accéder de façon optimisée à l'ensemble des techniques et expertises mises à disposition.
Procédure d’accueil des nouveaux projets
Tout nouveau projet doit être présenté lors d’une réunion ATLAN (hebdomadaire, le lundi de 14h à 15h30, en salle RDJ ou en visioconférence).
NB : ces réunions ne sont pas exclusivement dédiées aux nouveaux projets. Elles peuvent également servir à échanger sur vos projets en cours, poser des questions ou solliciter des conseils.
Merci d’envoyer un mail à atlan@univ-nantes.fr afin de convenir ensemble d’un créneau de 30 minutes pour la présentation de votre projet.
Avant la réunion, nous vous invitons à compléter la fiche « NouveauProjetATLAN.pdf », disponible dans le dossier UNCLOUD et sur RMES_ECHANGE.
Aucun projet ne pourra être lancé sans le respect de ces étapes.
Our laboratory has set up and harbors 6 technical platforms equipped with large and medium-size equipment and managed by dedicated core lab technical staff.
SC3M: electron Microscopy, Microcharacterization and functional Morphohistology and imaging
(Scientific coordinator: M-A Boutet ; Deputy scientific coordinator G Mabilleau ; Technical coordinators: J. Veziers and Y Le Guennec)
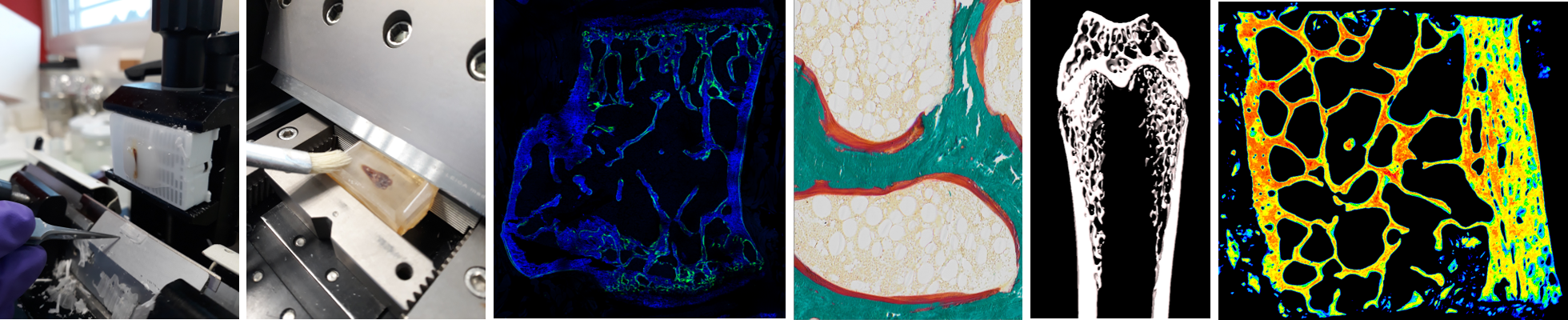
This core facility gathers in one location large equipments for transmission electron microscopy (TEM), scanning electron microscopy (SEM) and electron-probe by EDX energy dispersion, microtomography, confocal microscopy and FTIR mapping, as well as equipment for histology sample preparation (ultramicrotomy, critical point apparatus, cryomicrotome, gold/palladium sputtering, carbon sputtering). We also recently invested in a CatWalk equipment from Noldus company, a highly sensitive automated tool to assess gait and locomotion of rodents through the recording of their footprints when they voluntarily traverse a glass plate.SC3M is member of SFR Sante F. Bonamy.
More information on our expertise on Imaging / Histology

The molecular histology platform HiMolA offers its service in three main type of activities:
• Investigation of bone microarchitecture and histomorphometry: HiMolA performs conventional bone histomorphometry, including bone preparation (calcified and decalcified), embedding in polymethylmethacrylate or paraffin, sectioning, and staining (HPS, toluidine blue-borax, tartrate-resistant acid phosphatase, Goldner trichrome, Perl’s and solochrome azurine). HiMolA has also expertise in static and dynamic histomorphometry measurements according to the American Society for Bone and Mineral Research Guidelines. The platform is also specialized in X-ray imaging of calcified tissue by micro/nanoCT and propose several solutions ranging from in-vivo investigation of bone microarchitecture in small animals to ex-vivo analysis of specimen in three dimensions (3D).
• Investigation of extracellular matrix material properties: HiMolA has developed a strong expertise in the assessment of bone material properties by several methodologies ranged from (i) quantitative backscattered electron imaging (qBEI), dedicated to the study of mineralization pattern of bone samples and other mineralized tissues, (ii) to vibrational microscopies (Fourier transform infrared microspectroscopy and imaging, Raman microspectroscopy) for the study of organic and mineral moieties of extracellular matrices.
• Biomechanical investigation: HiMolA also proposes its services to the evaluation of biomechanical response of a variety of samples ex-vivo at the macroscopic (three-point bending, compression test, elongation test) or micro/nanoscopic (nanoindentation) levels.
Site web: https://regos.univ-angers.fr/en/platforms/himola.html
Contact email: himola@univ-angers.fr
Charter of use
BIO3 platform : Biomaterials, Biofabrication, Biomechanics facility

The BIO3 platform supports skeletal surgery and regenerative medicine projects in three research areas:

- Biomaterial synthesis: two separate fully equiped laboratories (orbital mixer, planetary ball mill, rotor mill, mortar grinder, sieving tools, chemical reactors, dialysis devices, freeze dryers, isostatic press, hight T° furnaces) allow BIO3 users to synthesise CaP ceramics, inorganic or organic (including hydrogels, fibers, etc…) biomaterials without cross-contamination. These materials can be used as bio Ink and be translate to biofabrication.

- Biofabrication through electrospinning and additive manufacturing: from CAD (Meshmixer, Solidworks) to printing (FDM, pneumatic extrusion, electrowriting), BIO3 users conduct their projects with the platform's technical expertise and brand new printers from Ultimaker, Cellink and RegenHU compagnies.

Sc4Bio

Cell culture ► Boris HALGAND
Molecular biology ► XXX
INOA ► Sophie ALLAIN
Bioinformatic ► in progress

Training is made to all new users by teaching the basics of cell culture in one week. It includes ways of working under class II Laminar Flow Hoods, cell passage and counting and specific technics referring to the research program of the new user.
The support part goes from technical help during experiments to experiment design, scientific watch, and development of new technics, especially in the field of bone, cartilage, tendon, teeth/gingiva, intervertebral disc, and synovium. Both cell lines and primary cell cultures are mastered, from humans and animals (mouse, rat, sheep etc). Differentiation tests are performed (1) from mesenchymal stem cells toward osteoblasts, chondrocytes and adipocytes, and (2) from monocytes toward osteoclasts and macrophages. Direct and indirect co-cultures are also performed, as well as 3D organoids, using tissue explants, biomaterials (hydrogels, calcium phosphate ceramics), extracellular vesicles, and various drug/gene delivery systems.
Logistics of the cell culture facility is also performed, permitting users to focus solely on their experimentations.
The different cell culture rooms (L1 and L2) contain all the basic culture materials such as incubators, laminar flow hood, centrifuges, and liquid nitrogen storage equipment for cell preservation. It also offers the possibility of cell culture under hypoxia (INVIVO400 system) and to perform 3D bio-printing of live cells (regenHU system, under BIO3 platform supervision).
(Scientific coordinator: F. Jehan ; Technical coordinator : xxx )
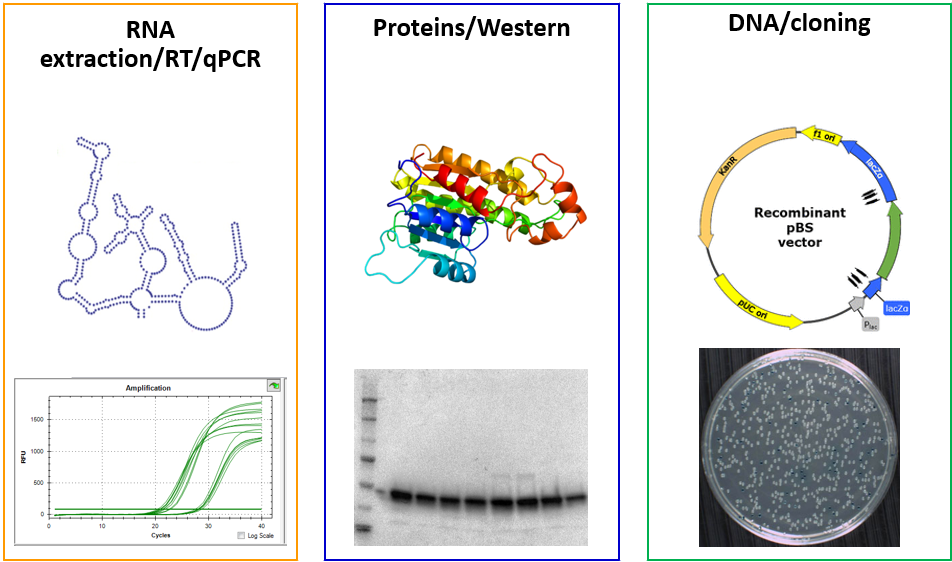
Activity on the platform is divided into three modules: RNA extraction/RT/qPCR, Proteins/Western, and DNA/cloning. All modules are spread in several labs to limit the possible contamination of biological materials and respect the rules of hygiene and safety.
Users have access to various equipments such as a nucleic acid extractor robot, the Tristar plate reader, a Nanodrop, various gel apparatus for proteins or nucleic acids analysis, PCR and real-time qPCR apparatus, etc…, as well as the appropriate consumables and kits.
To help laboratory users in their research projects, the molecular biology platform offers training in different fields of molecular biology and proteomics (Nucleic acid extraction, qPCR, Western Blot, cloning, CRISPR, and other techniques), and in carrying out and analyzing experiments. Training is performed by the technical coordinator of the platform.
(Scientific coordinator: F. Blanchard; Technical coordinator: S. Allain)
The INOA facility offers scientific and technical support for experiments related to the characterization of skeletal cell responses (synovial fibroblasts, osteoblasts, macrophages, osteoclasts for example), particularly inflammatory ones. We are located on the 1st floor of the Veil building of the Faculty of Medicine.
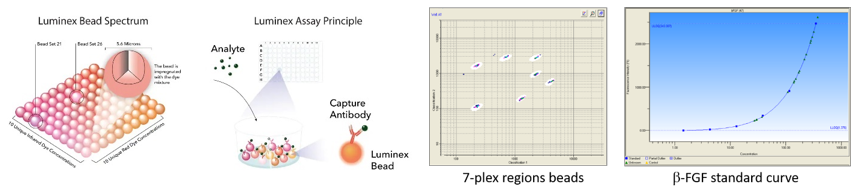
- ELISA/Luminex cytokine assays: we provide ELISA/Luminex (up to 50 analytes simultaneously) kits for quantification of cytokines/growth factors in serum, synovial fluid, or cell culture supernatant. After training, members of the RMeS lab have free access to ELISA/Luminex equipments.
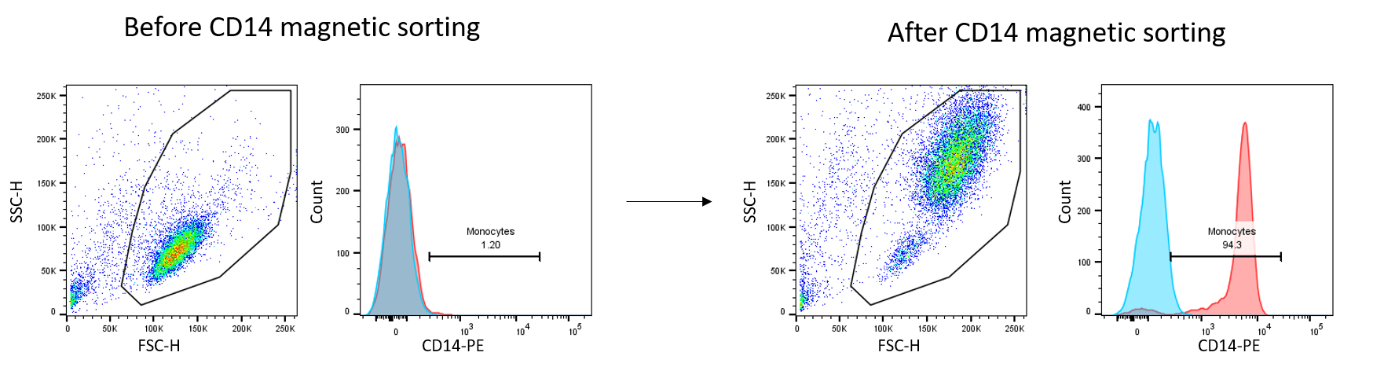
- Tissue dissociation: we have access to a tissue dissociator (gentleMACS™ Octo Dissociator) to individualize cells for culture, characterization by flow cytometry or single cell transcriptomics for example. Our expertise ranges from dissociation of synovial membrane or tendon to adipose tissue and cartilage.
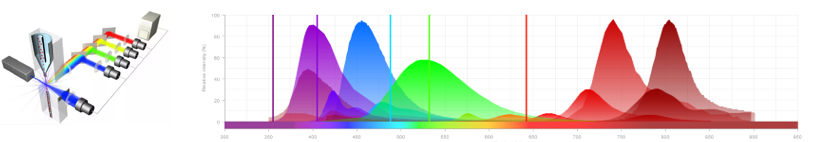
- Cellular characterization: we offer support for the development of antibody panels, realization, acquisition, and analysis (FlowJo software) of phenotypic staining by flow cytometry. We have access to Cytocell platform cytometers, including Canto II (3 lasers 405/488/635nm, 8 detectors), and Symphony (5 lasers 355/405/488/561/633nm, 30 detectors).
- Cell culture: we provide training and access to different cell culture rooms (L1 and L2) containing all the basic culture materials such as incubators, laminar flow hoods, centrifuges etc.