REGOS
Regenerative medicine of bone tissues
This team will be composed of 56 people (including PhD students) corresponding to a 34 Full-Time Equivalent including 25 senior researchers with Research Supervisor Qualifications (HDR in French format) and will be headed by P. Weiss with V. Geoffroy as deputy head. This team will involve 2 Inserm senior scientists, 1 Atip-Avenir researcher, 27 University/Hospital researchers, 7 University/ONIRIS researchers, 3 postdoctoral researchers, 4 technical staff and 12 PhD students.
The REGOS team is a translational and transdiciplinary team that will focus on the pathophysiology and aging of bone tissues from their basic aspects (aging, cell-cell communication, tissue homeostasis, inflammation) to more applied aspects (osteoporosis, periodontitis, irradiated bones, hypotrophic tissues…). This team will also support the development of hybrid biomaterials (ceramics, cements, hydrogels) and functionalization approaches to better exploit the properties of MSC, extracellular vesicles and modulators of gene expression (ie microRNA) and new innovative medicines designed to enhance bone quality for the regenerative medicine of bone and dental tissues.The REGOS team is divided into 5 groups from the basic science to the medical application: Molecular control of bone aging and bone regeneration (EPIGEN, Group 1), Skeleton-Muscle-Intestine crosstaLk and innovative thErapeutics (SMILE, Group 2), Inflammatory mediators and microenvironment in bone and teeth (INFLAMED, Group 3) Scaffolds for maxillo-facial bone augmentation (SAMBA, Group 4), Translational research in calcified tissue regeneration (MATOS, Group 5)
Funding Bodies
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
Partners
 |
 |
 |
 |
Group 1 Epigen : Molecular control of bone aging and regeneration (EPIGEN)
Leader: V. Geoffroy
This group involves 2 senior scientists (V. Geoffroy DR2 Inserm, F. Jehan CRCN Inserm), and currently 1 post-doctoral fellow ( 
This group deals with the fundamental processes controlling aging, degenerescence and the process of bone regeneration. This group aims to investigate novel mechanisms of osteoformation and find target molecules that could contribute to reprogram osteogenic cells.
Our group seeks to mimic the major molecular mechanisms controlling bone aging and the ability of bone to self-repair in order to induce/enhance bone regeneration. For this, we propose to identify regulators of bone formation whose activity decreases or increases with aging, such as
1) microRNAs or their target genes which could be used as molecular therapies in bone repair (miRbone project), and 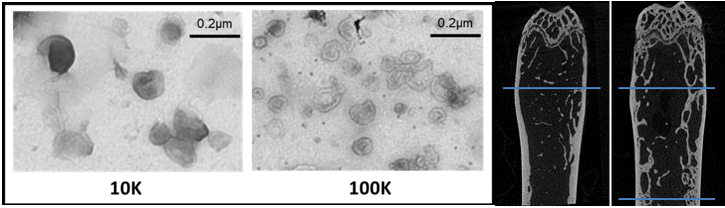
2) extracellular vesicles cargos that may be essential in communication pathway between bone cells and that mimic the major processes of physiological bone repair that are driven by osteocytes (OVERbone project).
Our long-term goal is to manipulate these regulators with the clinical aim of (i) restoring bone mass and balance formation/resorption during aging, and (ii) proposing new therapeutic strategies of bone repair in combination with biomaterials, through the mobilization and activation of the healing potential of the host tissue.
Return
Group 2 SMILE : - Skeleton-Muscle-Intestine crosstaLk and innovative thErapeutics
 Leader: G. Mabilleau
Leader: G. Mabilleau
This group involves 9 University/Hospital Professors or assistant-professors B. Bouvard (PU-PH), J-D Kün-Darbois (PU-PH), E. Legrand (PU-PH), H. Libouban, (PU), G. Mabilleau (MCU-PH), L. Rony (MCU-PH), L.Hubert (PH), F Robin (MCU-PH), T Moubib (CCA), 2 PhD student G. David, L. Fontaine, 2 engineer, C. Malory, S Gharbi and 2 Masters students.
Our group is interested in understanding how several peripheral tissues may control bone remodelling and in developing new therapeutics to fight against bone fragility that occur with ageing or with metabolic disorders. Our approach consists in targeting bone quality and especially bone ECM material properties rather than bone mass. This approach is innovative and in rupture with actual therapeutic.
Our main objectives are:
1) To better understand the role of the gut and muscle tissues in controlling bone strength and quality
2) To develop new mimetics based on gut hormones and myokines for the prevention and treatment of bone fragility https://regos.univ-angers.fr/en/index.html
Group 3 INFLAMED : - Inflammatory mediators and microenvironment in bone and teeth
Leader: A. Gaudin
This group involves 8 University/Hospital Professors or assistant-professors (P. Lesclous (PU-PH), C. Nich (PU-PH), A. Gaudin (PU-PH), A. Cloitre (MCU-PH), E. Hascoët (MCU-PH) Y. Maitre (MCU-PH) E. Renard (PU-PH), S. Serisier (MCU-PH), 2 PhD student (A Louisy, J Jamoneau), 1 engineer (S. Tessier).
The inflammatory microenvironment particularly immune cells plays acrucial rol at the tissue injury/damage site to establish and orchestrate proregenerative milieu. Different types of immune cells and several Inflammatory mediators participate in all the phases of tissue repair/regeneration and homeostasis. This group aims to identify therapeutic targets of interest in the treatment of periodontitis (Program 1) , to multi-functionalize drug delivery systems in order to promote resolution of inflammation in pulpitis and periodontitis context (Program 2) and to mitigate bone resorption and joint implant loosening related to wear debris particles (Program 3) . Although different by nature, these 3 programs will contribute to the effort of the other REGOS groups to produce data from physiopathology of bone/pulp inflammation to 4R (Replace, Repair, Regenerate, Reprogram) medical treatments.
Program 1. Identification of therapeutic targets in alveolar bone resorption (Leader: A. Cloitre, Nantes)
O 
Program 2. Drug delivery systems and resolution of inflammation (Leader: A. Gaudin, Nantes)
Project 1. New biphasic microgel as lipoxin delivery system for tissue engineering
Lipoxins regulate functions of the innate immune system (attenuate monocyte recruitment and induce a pro-resolving macrophages phenotype), inhibit functions of dendritic cells (DC) and also modulate the adaptive immune system by decreasing memory B-cell responses. The major challenge in using lipoxins or analogs is their chemical instability a feature that impairs their use for treating inflammatory diseases or tissue lesions. One goal of this project is to evaluate the potential benefits of a new Biphasic Microgel (BPMG) of Si-HPMC as LXA4 delivery system.
Project 2. Effect of inflammation on dental mesenchymal stem cells derived-exosomes - Application to pulpitis treatment and dental pulp regeneration
MSC-derived EVs immunomodulatory functions have already been described and include suppression of M1 macrophage polarization and enhancement of M2 macrophage activation. The specific goal of this project is to test the hypotheses that 1) in vitro simulated inflammation can modulate the immunosuppressive paracrine activity of DPSC/SCAPs and could therefore play a role in pulp regeneration, 2) MSC-derived EVs has immunomodulatory functions that can be manipulated to influence macrophage polarization in vitro and ultimately regeneration in vivo, and 3) Production of EVs from DPSC/SCAPs can be stimulated under simulated inflammatory conditions (preconditionned cells) in order to be tested for pulp regeneration.
 Program 3. Inflammation associated to particle-induced osteolysis (Leader: C. Nich, Nantes)
Program 3. Inflammation associated to particle-induced osteolysis (Leader: C. Nich, Nantes)
In the field of artificial joints, inflammation, caused by infection of the implant or by particles issued from the bearing couple, may lead to local bone resorption. This will result in loss of implant fixation and loosening in up to 15% of patients in the decade following a total hip or knee arthroplasty. Various biological strategies targeting macrophage are under consideration to mitigate particle-induced bone resorption. Hence, selective antagonists of the estrogen receptors or of the cannabinoid receptors have been shown to limit bone resorption in experimental models of particle-induced osteolysis (OL). Recently, the human beta-defensin (HBD), a cationic peptide with immunomodulatory effects on innate immune response, has been shown to suppress the production of TNF-alpha and IL-6 in murine LPS-activated macrophages. However, there is still no consensus on the optimal therapeutic combination to alter these inflammatory processes. Therefore, we aim to compare the effect of ER antagonists, CBDR antagonists and HBD in an experimental model of particle-induced OL. These results will help define future therapeutic targets for the mitigation of wear-debris particle-induced OL, and more generally, of inflammation of calcified tissues.
Return
Group 4 SAMBA : - Scaffolds for maxillo-facial bone augmentation
This group involves 1 ATIP-Avenir junior group leader (B. Charbonnier), 1 reasherch engineer (V. Biscaccianti ) 1 PhD student (P. Quenum) and will recruit within a year 2 master student
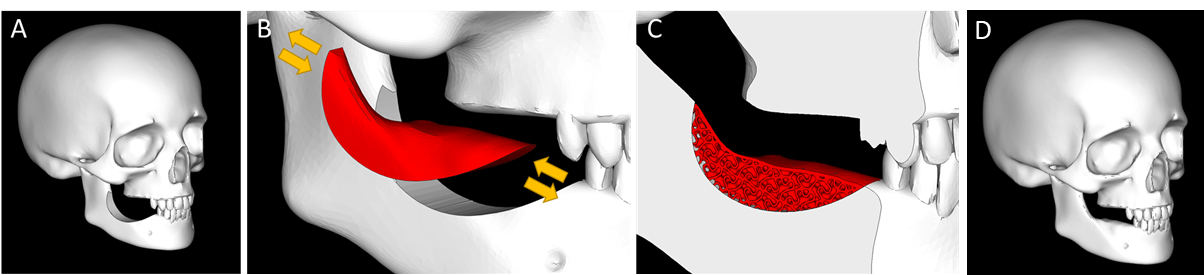
Missing teeth constitutes a significant healthcare and socioeconomic burden, especially in the senior population. Today, dental implants are considered the most adequate solution to restore physiological functions, aesthetics and avoid further oral degradation. However, their placement requires a sufficient bone volume, and mandibular bone augmentation procedure is required in more than 50% of the cases.
SAMBA relies on the development of a time-evolutive patient-specific scaffold for the personalized augmentation of atrophic mandibular bone; and this to democratize such interventions even in small practices with limited staff and equipment. Aims includes (i) the development and characterization of innovative materials stimulating the formation of a new vascularized tissue in compromised environment, (ii) the development of dedicated additive manufacturing devices for the robust and rapid and sterile production of human-sized scaffolds, and (iii) the in vivo evaluation of the potential of such scaffold to answer the targeted clinical unmet need.
Return
Group 5 MATOS : - Translational research in calcified tissue regeneration
Leader: P. Weiss
This group involves 17 University/Hospital Professors or assistant-professors : N. Bouhsina (MCU), C. De fourmestraux (MCU), H. Gautier (PU), V.Trichet (PU), P. Blery (MCU-PH), P. Maitre (MCU), G. Rethore (MCU-PH) X. Struillou (MCU-PH), O. Gauthier (PU), AG. Chaux (PU-PH), P. Corre (PU-PH), F. Espitalier (PU-PH), L. Le Guehennec (PU-PH), O. Malard (PU-PH), P. Perrot (PU-PH), A. Soueidan (PU-PH), P. Weiss (PU-PH), 2 post-doctoral fellows (C Mayslich, P. Guihard), currently 7 PhD students (C. Béchina, S. Bertin, C.Hachimi Alaoui, U Lancien,, C Rota, R Saadan, T Serhal).
The Group 4 in the REGOS team is a translational group focused on bone regeneration research with all the aspects related to this topic from physiopathology to 4R medical treatments (Replace, Repair, Regenerate, Reprogram). Our research areas are bone apposition on mandible and bone regeneration in periodontitis or peri implantitis, bone regeneration after irradiation for cancer treatment, bone reconstruction in cleft and palate syndrome and long bone interruptive lesions. Our projects are divided into 4 programs from the basic science to the medical application: 1) Hybrid biomaterials for bone scaffolds 2) Translational research in irradiated bone regeneration 3) Translational research in personalized bone repair and 4) Translational research in periodontal and periimplantitis bone regeneration.

Program 1. Hybrid Biomaterials for bone scaffold (Leader: P. Weiss)
Program 2. Translational research in irradiated bone regeneration (Leader: F. Espitalier)
Considering the high rate of MSC mortality after implantation with material in bone defects, due to local ischemia or immunoreaction, we propose an innovative strategy combining MSCs embedded in a low resorbable hydrogel made of a self-crosslinking polymer (silanized HPMC) that will be mixed with in a new high resorbable self-crosslinking injectable bone substitute made of Silanized Hyaluronic Acid (IXBONE project). This formulation will offer an injectable bone substitute with active protection of MSCs that could deliver specific bioactive factors for the regulation of inflammation and osteo-induction processes in local environments.
Program 3. Translational research in personalized bone repair (3D) (Leader: P. Corre)
Jaw bone defects are mainly caused by malformations such as cleft lip/palate at the maxilla, and tumor removal at the mandible. Autologous bone grafting with non-vascularized graft and fibula free flap represent the gold standard in alveolar cleft repair and large mandibular defects respectively. Innovative formulations and extrusion devices will be used to design 3D printed nano-, micro- and macro-textures with 3D specific printed designs. We plan to use tunable hydrogels in association to 3D printed customized composite calcium phosphate cement to prepare specific, non-fragile, ductile cement that can be bioprinted for many personalysed 3D construct in bone regeneration (GIJaw project). These innovations will allow developing smart surgical guides and osteosynthesis devices for jaws surgery.
Program 4. Translational research in periodontal and periimplantitis bone regeneration (Leader: A. Soueidan)
Despite being the most effective treatment method for replacing missing teeth, Ti-based dental implants are associated with diseases such as peri-implantitis which are caused by bacteria adhering and proliferating on the implant surface and might result in implant loss. We plan to use foam cements in association with different calcium phosphate nano particles dopped with magnesium, strontium or gallium in collaboration with latvian university (J. Locs) and Laval University (D. Mantovani) for specific mechanichal and biological properties (ERA-NET Euronanomed III NanoImplant project). We will develop an innovative ready-to use composite-nano drug delivery systems for one-shot effective treatment of peri-implantitis based on an easily applicable construct composed of nano-hydroxyapatite (nHAp) particles immobilized within Ti-adhesive silanized hyaluronic acid hydrogel. With this approach, the physiological wound healing process will be supported by preventing the ingrowth of the epithelial/connective soft tissues and promoting osseous regeneration around implant and by the delivery of antibacterial Ga3+ ions and/or antibiotics incorporated in nHAp.




